
Classified as a transition metal, Cobalt is a solid at room temperature. Exposure to cobalt-60, a powerful gamma ray emitter, may cause cancer. Cobalt is a chemical element with symbol Co and atomic number 27. For the first two, also include the orbital notation. Cobalt should be handled with care because of its toxicity and its risk factor in nuclear confrontation. Electron Configuration Practice Worksheet Write the electron configurations of the following elements. Argon is a noble gas, which means its outer shell (3p) is filled. In small amounts, cobalt is an essential element for humans and many other living organisms, and it is also a central component of vitamin B-12 or cobalamin. The electron configuration of cobalt is 1s2 2s2 2p6 3s23p6 3d7 4s2. The valence electrons of these elements range.
#Electron configuration of cobalt series#
The elements in the lanthanides and actinides series are called f-block elements. Therefore, the valence electrons of cobalt are nine.

If you look at a series of elements, cobalt is in. For example, the electron configuration of cobalt shows that the electron configuration ends in a d-orbital and that the last shell has a total of nine electrons.
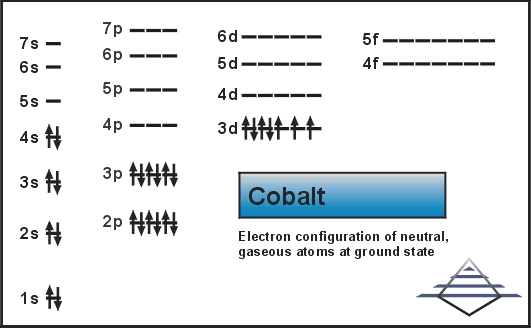
Brandt between 17 when he was able to show that cobalt colors glass a rich blue. Cobalt has fifteen electrons in its third shell that holds a maximum of eighteen electrons (as seen in zinc). This solid ferromagnetic silver-white element was known in ancient times for its compounds, but its discovery was credited to G. Obtained from: arsenic, oxygen, sulfur, cobatineįrequently, cobalt is associated with nickel because both elements have characteristic ingredients of meteoric iron.


 0 kommentar(er)
0 kommentar(er)
